Eliminating a Bad Actor in Cancer: Nurix’s Targeted Cancer Therapy Programs
BTK Degraders
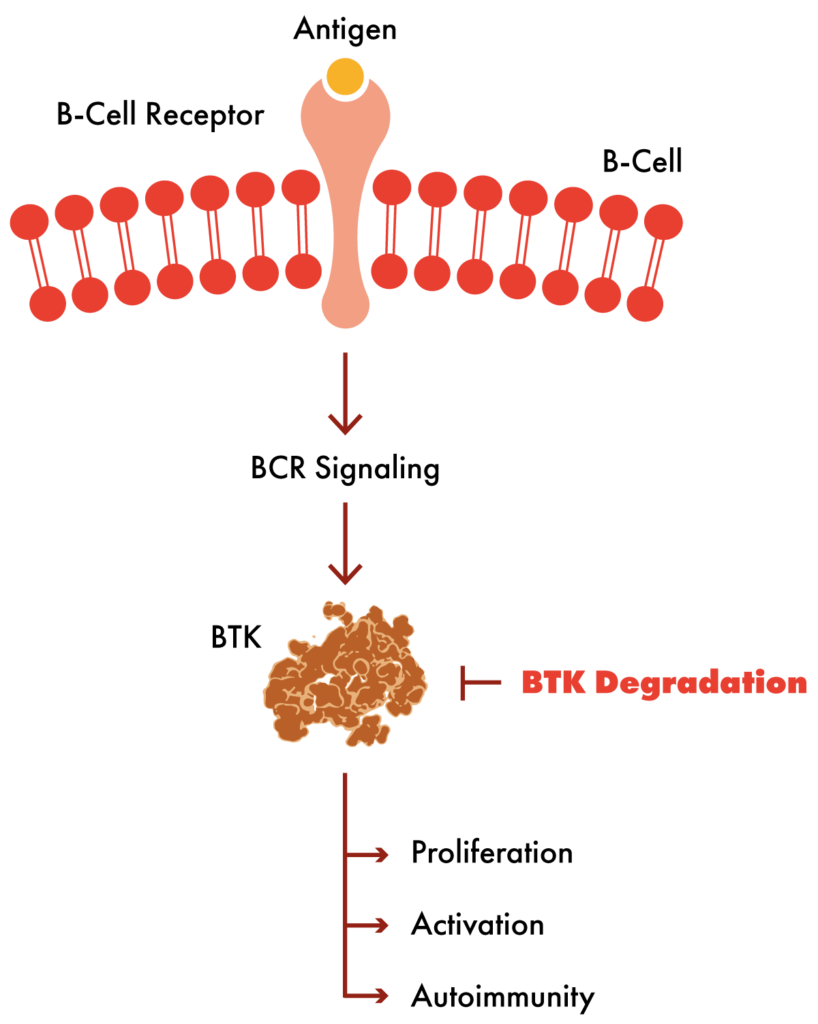
The U.S. Food and Drug Administration and global regulatory agencies have approved several inhibitors of Bruton’s tyrosine kinase (BTK), which are now the standard of care across multiple tumor types. We believe that removal of the BTK protein through Targeted Protein Degradation could generate superior outcomes for patients who no longer respond to or do not tolerate current therapies, including patients whose tumors have acquired resistance to current therapies through mutations in the BTK protein.
BTK is a validated clinical target for the treatment of hematologic malignancies and autoimmune diseases. BTK is a master regulator of B-cell activity, and inhibition of BTK results in improved clinical outcomes for patients with a variety of B-cell mediated cancers such as chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma (NHL).
We have developed two unique and functionally distinct BTK degraders that harness cereblon (CRBN), an E3 ligase active in hematopoietic cells. Cereblon itself is the target for a class of drugs with immunomodulatory activity, including lenalidomide and pomalidomide that induce cereblon to degrade so-called neosubstrates Ikaros (IKZF1) and Aiolos (IKZF3). We have designed two BTK degraders with and without the ability to induce neosubstrate degradation, creating two functionally distinct drug candidates to address the unmet medical needs across indications and lines of therapy.
| Drug Program | MOA | Target/Delivery | Therapeutic Area | Discovery - Lead Op | IND enabling | Phase 1a | Phase 1b |
|---|---|---|---|---|---|---|---|
| Hematology/Oncology | |||||||
| NX-5948 | TPD | BTK | B-cell malignancies |
| |||
| NX-2127 | TPD | BTK + IKZF | B-cell malignancies |
| |||
| NX-1607 | TPE | CBL-B | Immuno-oncology |
| |||
| Multiple | TPD | Undisclosed | Undisclosed |
| |||
| Multiple | TPD | Undisclosed | Undisclosed |
| |||
| Multiple | TPD | Undisclosed | Undisclosed |
| |||
| Multiple | DAC | Undisclosed | Oncology |
| |||
| Drug Program | MOA | Target/Delivery | Therapeutic Area | Discovery - Lead Op | IND enabling | Phase 1a | Phase 1b |
| Inflammation & Immunology | |||||||
| NX-5948 | TPD | BTK | Inflammation/autoimmune |
| |||
| NX‑0479/GS‑6791 | TPD | IRAK4 | Rheumatoid arthritis and other inflammatory diseases |
| |||
| STAT6 degrader | TPD | STAT6 | Type 2 inflammatory diseases |
| |||
| Undisclosed | TPD | Undisclosed | Inflammation/autoimmune |
| |||
| Hematology/Oncology | NX-5948 - TPDBTK - B-cell malignancies |
Preclinical IND-Enabling Phase 1a Phase 1b |
|
| NX-2127 - TPDBTK + IKZF - B-cell malignancies |
Preclinical IND-Enabling Phase 1a Phase 1b |
|
| NX-1607 - TPECBL-B - Immuno-oncology |
Preclinical IND-Enabling Phase 1a Phase 1b |
|
| Multiple - TPDUndisclosed - Undisclosed |
Preclinical IND-Enabling Phase 1a Phase 1b |
|
| Multiple - TPDUndisclosed - Undisclosed |
Preclinical IND-Enabling Phase 1a Phase 1b |
|
| Multiple - TPDUndisclosed - Undisclosed |
Preclinical IND-Enabling Phase 1a Phase 1b |
|
| Multiple - DACUndisclosed - Oncology |
Preclinical IND-Enabling Phase 1a Phase 1b |
|
| Inflammation & Immunology | NX-5948 - TPDBTK - Inflammation/autoimmune |
Preclinical IND-Enabling Phase 1a Phase 1b |
|
| NX‑0479/GS‑6791 - TPDIRAK4 - Rheumatoid arthritis and other inflammatory diseases |
Preclinical IND-Enabling Phase 1a Phase 1b |
|
| STAT6 degrader - TPDSTAT6 - Type 2 inflammatory diseases |
Preclinical IND-Enabling Phase 1a Phase 1b |
|
| Undisclosed - TPDUndisclosed - Inflammation/autoimmune |
Preclinical IND-Enabling Phase 1a Phase 1b |
|
NX-5948 (BTK degrader for B-cell malignancies)
NX-5948 is an oral small molecule degrader of BTK. Preclinical data support the activity of NX-5948 in tumor models harboring either wild-type BTK or BTK with mutations conferring clinical resistance to FDA approved agents. NX-5948 has demonstrated the ability to cross the blood brain barrier in animal models creating potential therapeutic utility in central nervous system (CNS) lymphoma and other diseases in the brain. NX-5948 has also demonstrated activity in animal models of autoimmune disease including models of rheumatoid arthritis and multiple sclerosis.
NX-5948 is being tested in an ongoing Phase 1 trial for patients with B-cell malignancies who have failed prior treatments.
NX-2127 (BTK + IKZF degrader for B-cell malignancies)
NX-2127 is an oral small molecule degrader of BTK and cereblon neosubstrates IKZF1 (Ikaros) and IKZF3 (Aiolos). Cereblon immunomodulatory drugs that induce degradation IKZF1 and IKZF3 such as lenalidomide and pomalidomide are FDA approved for a variety of hematologic malignancies including multiple myeloma, follicular lymphoma, and mantle cell lymphoma. We hypothesize that the combination of BTK degradation and cereblon immunomodulatory activity will have enhanced therapeutic benefit in patients suffering from a variety of B-cell malignancies.
Initial clinical data support the activity of NX-2127 in patients whose tumors harbor either wild-type BTK or BTK with mutations conferring clinical resistance to FDA approved agents. Initial clinical data also confirm potent BTK degradation with once daily oral dosing. NX-2127 is being tested in an ongoing Phase 1 trial for patients with B-cell malignancies who have failed prior treatments.


 '
'