Building Immunity to Cancer: Nurix’s Immuno-Oncology Program
Targeted Protein Elevation: CBL-B Inhibition
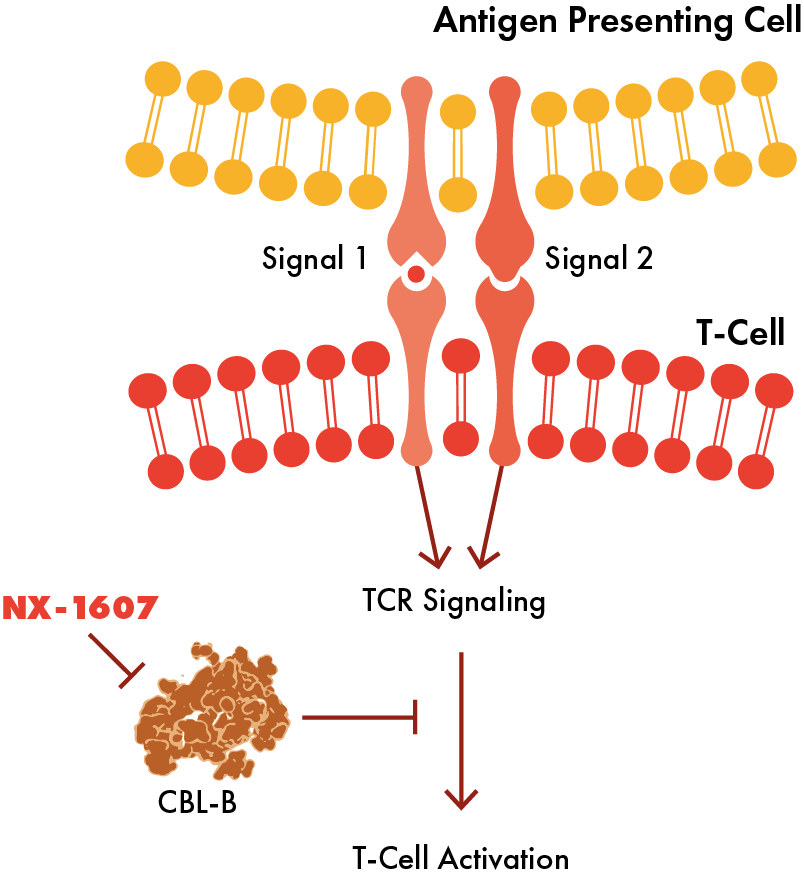
Casitas B-lineage lymphoma proto-oncogene-b (CBL-B) is an E3 ligase that is expressed in immune cells and in the context of cancer, acts as a brake on the immune system. CBL-B functions as an intracellular checkpoint that negatively regulates T cell activation, NK cell activity, and immune response through degradation of specific inhibitory signaling proteins.
Inhibition of CBL-B with a small molecule drug activates T and NK cells, a goal in the treatment of cancer, where T cells and the entire immune system can be mobilized to destroy a tumor.
| Drug Program | MOA | Target/Delivery | Therapeutic Area | Discovery - Lead Op | IND enabling | Phase 1a | Phase 1b |
|---|---|---|---|---|---|---|---|
| Hematology/Oncology | |||||||
| NX-5948 | TPD | BTK | B-cell malignancies |
| |||
| NX-2127 | TPD | BTK + IKZF | B-cell malignancies |
| |||
| NX-1607 | TPE | CBL-B | Immuno-oncology |
| |||
| Multiple | TPD | Undisclosed | Undisclosed |
| |||
| Multiple | TPD | Undisclosed | Undisclosed |
| |||
| Multiple | TPD | Undisclosed | Undisclosed |
| |||
| Multiple | DAC | Undisclosed | Oncology |
| |||
| Drug Program | MOA | Target/Delivery | Therapeutic Area | Discovery - Lead Op | IND enabling | Phase 1a | Phase 1b |
| Inflammation & Immunology | |||||||
| NX-5948 | TPD | BTK | Inflammation/autoimmune |
| |||
| NX‑0479/GS‑6791 | TPD | IRAK4 | Rheumatoid arthritis and other inflammatory diseases |
| |||
| STAT6 degrader | TPD | STAT6 | Type 2 inflammatory diseases |
| |||
| Undisclosed | TPD | Undisclosed | Inflammation/autoimmune |
| |||
| Hematology/Oncology | NX-5948 - TPDBTK - B-cell malignancies |
Preclinical IND-Enabling Phase 1a Phase 1b |
|
| NX-2127 - TPDBTK + IKZF - B-cell malignancies |
Preclinical IND-Enabling Phase 1a Phase 1b |
|
| NX-1607 - TPECBL-B - Immuno-oncology |
Preclinical IND-Enabling Phase 1a Phase 1b |
|
| Multiple - TPDUndisclosed - Undisclosed |
Preclinical IND-Enabling Phase 1a Phase 1b |
|
| Multiple - TPDUndisclosed - Undisclosed |
Preclinical IND-Enabling Phase 1a Phase 1b |
|
| Multiple - TPDUndisclosed - Undisclosed |
Preclinical IND-Enabling Phase 1a Phase 1b |
|
| Multiple - DACUndisclosed - Oncology |
Preclinical IND-Enabling Phase 1a Phase 1b |
|
| Inflammation & Immunology | NX-5948 - TPDBTK - Inflammation/autoimmune |
Preclinical IND-Enabling Phase 1a Phase 1b |
|
| NX‑0479/GS‑6791 - TPDIRAK4 - Rheumatoid arthritis and other inflammatory diseases |
Preclinical IND-Enabling Phase 1a Phase 1b |
|
| STAT6 degrader - TPDSTAT6 - Type 2 inflammatory diseases |
Preclinical IND-Enabling Phase 1a Phase 1b |
|
| Undisclosed - TPDUndisclosed - Inflammation/autoimmune |
Preclinical IND-Enabling Phase 1a Phase 1b |
|
NX-1607 (oral CBL-B inhibitor for solid tumors)
NX-1607 is an oral, small molecule CBL-B inhibitor with the potential to enhance innate and adaptive immune responses. In multiple preclinical animal tumor models NX-1607 demonstrated anti-tumor activity and prolonged survival. The combination of NX-1607 with an anti-PD-1 antibody provided additional benefit in animal models. The anti-tumor activity of NX-1607 is dependent on both CD8+ T cells and NK cells.
We are planning to develop NX-1607 in multiple solid tumors and lymphoma as monotherapy or in combination with other complementary therapies such as chemotherapy and potentially check point inhibitors. NX-1607 may also have utility in combination with cell therapies such as TIL or CAR T. A Phase 1 clinical trial of NX-1607 as a single agent therapy and in combination with paclitaxel chemotherapy in multiple oncology indications is ongoing.


 '
'