Protein dysfunction lies at the heart of numerous diseases.
While traditional therapies often focus on inhibiting problematic proteins, targeted protein degradation focuses on eliminating
disease-causing proteins.
By utilizing the cell’s natural protein degradation machinery, we are at the forefront of a medical revolution that has the potential to deliver safer, more profound, and longer-lasting treatments — and potentially cures — for challenging diseases.
How degraders work
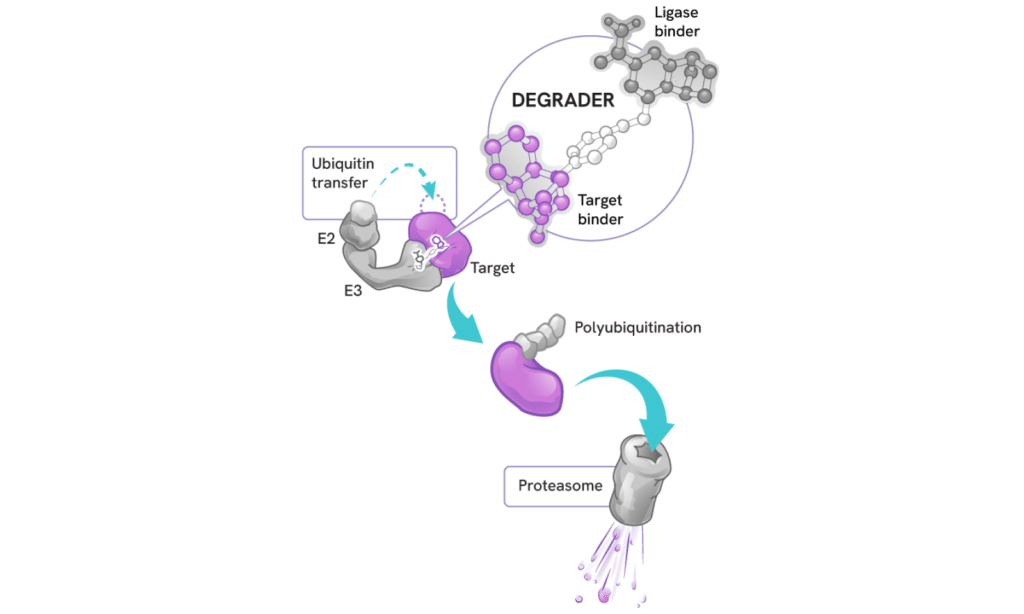
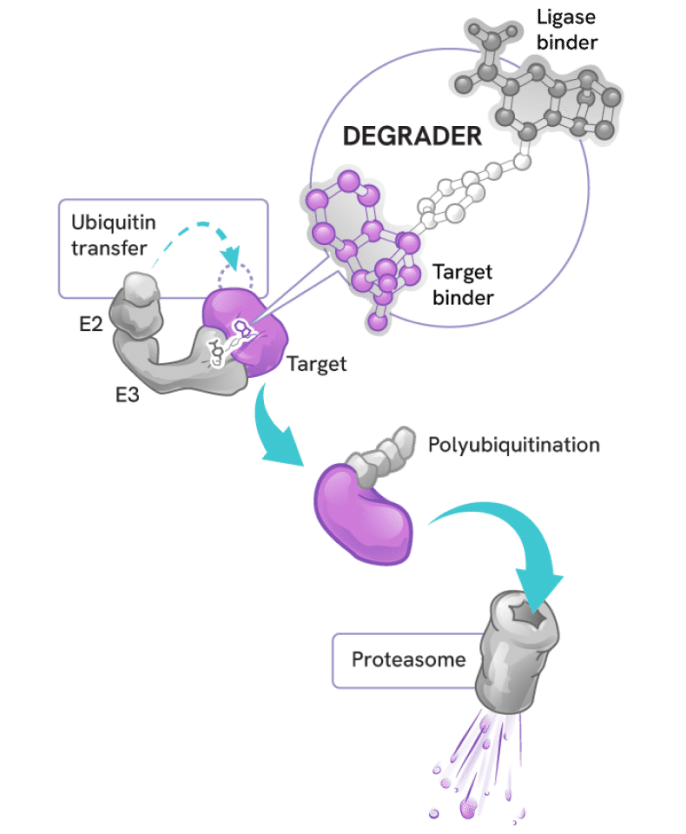
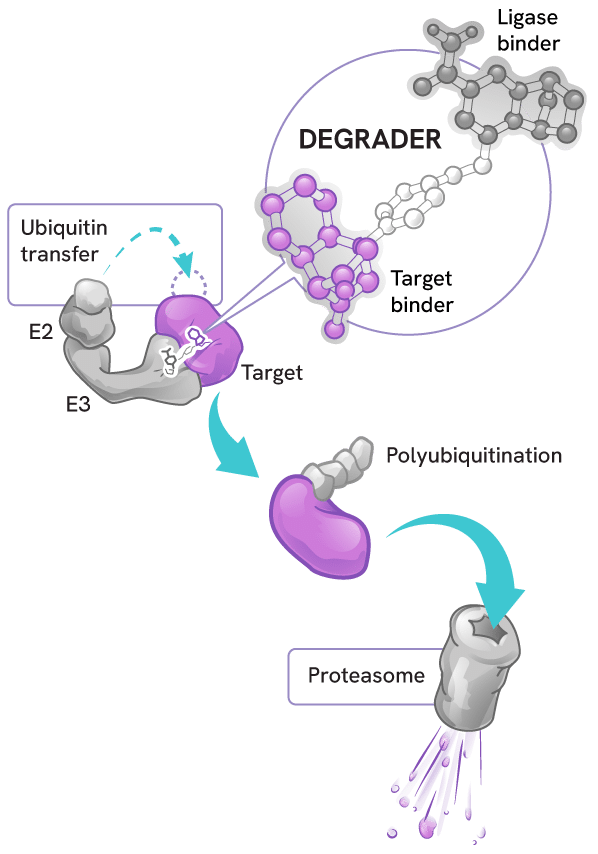
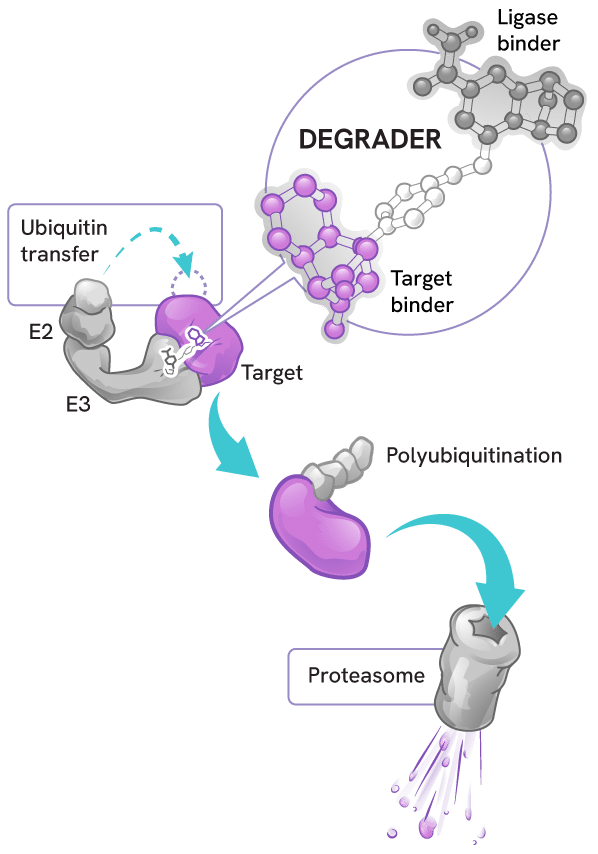
Degraders act as molecular matchmakers, bringing together two key players:
- An E3 ligase (a key part of a cell’s protein degradation machinery)
- A disease-causing target protein
This process, called induced proximity, enables the E3 ligase to tag the target protein with ubiquitin to mark it for disposal by the proteasome – the cell’s protein recycling center.
Given their ability to eliminate target proteins, degraders can achieve effects similar to genetic therapies that silence disease-causing genes. However, unlike genetic therapies that typically require infusion with genetic alteration or modulation, degraders are small molecule drugs that can be taken orally as a pill without genetic alterations.
Advantages of degraders
Degraders offer several key advantages over traditional small molecule inhibitors:
Overcoming Resistance
Degraders can maintain effectiveness against mutations that often render inhibitors ineffective, providing a critical advantage in treating evolving diseases such as cancer.
For example, certain kinases such as Bruton’s Tyrosine Kinase (BTK) can mutate, causing the kinase to lose its enzymatic function but gain a “scaffolding” function (serving as a structural support or framework that facilitates interactions between other proteins). This scaffolding mutation allows continued receptor signaling and cell growth despite impaired kinase activity.
The previously unappreciated scaffolding function of BTK is responsible for clinical resistance to enzymatic inhibitors, which Nurix elucidated in the publication of a manuscript in the journal Science. Our research demonstrated that degraders can address both the enzymatic and scaffolding functions of BTK in B-cell malignancies, potentially offering more comprehensive and durable treatment options.
Elimination of Dysregulated Proteins
By eliminating disease-causing proteins, degraders may provide longer-lasting and more effective therapeutic responses.
Catalytic Action
Each degrader can eliminate multiple target proteins, potentially allowing low drug levels to exert therapeutic effect.
Targeting The Undruggable
Over 80% of disease-related proteins are considered “undruggable” due to their lack of well-defined binding pockets and complex signaling interactions. Degraders overcome this limitation by leveraging a broader range of binding sites, enabling the elimination of previously undruggable proteins and opening new possibilities for treating intractable diseases.
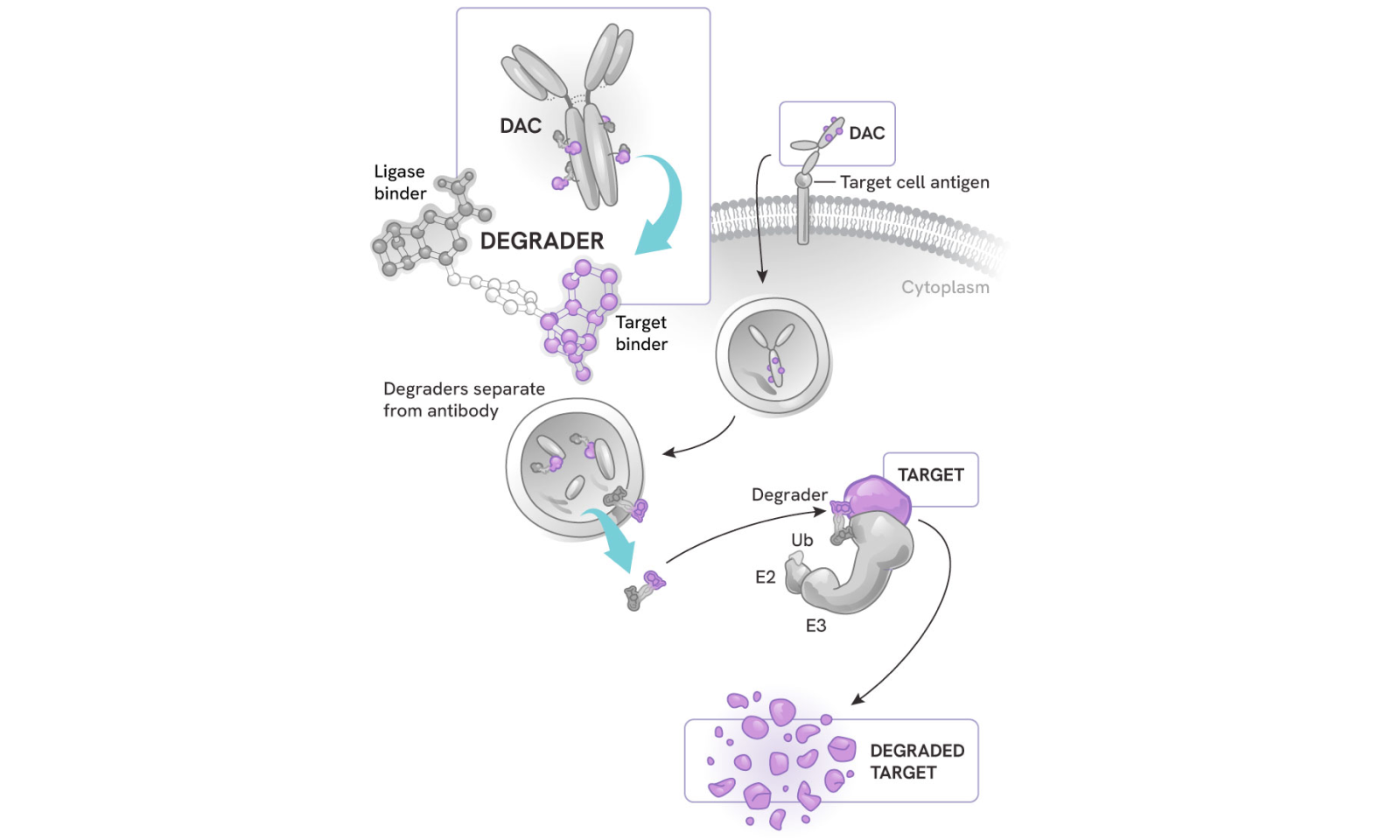
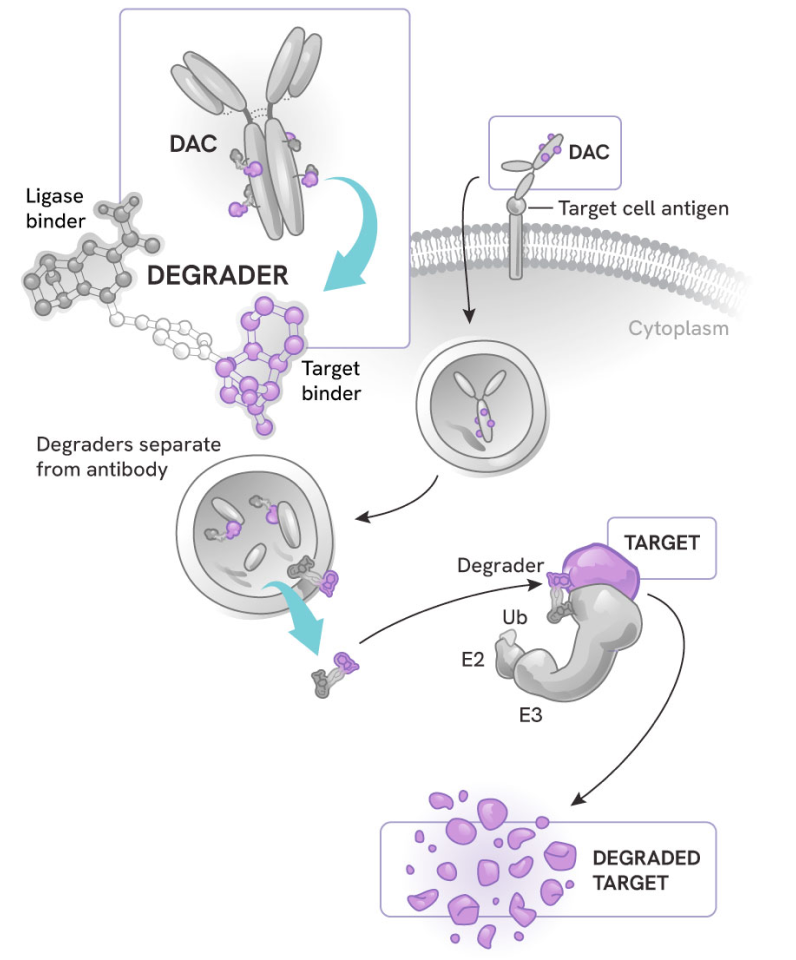
How degrader antibody conjugates (DACs) work
DACs represent the next evolution in antibody drug conjugates (ADCs) and targeted protein degradation, combining:
- The highly potent, selective, and catalytic activity of degraders
- The cell and tissue specificity of antibodies
The process:
- The antibody component binds to a specific antigen on the target cell surface, sparing healthy cells or other tissues
- The DAC is internalized into the cell
- The degrader small molecule is released into the cytoplasm, where it can precisely eliminate its protein targets
Advantages of DACs
DACs offer unique benefits over traditional antibody drug conjugates and build on the unique properties of degraders:
Improved Safety Profile
Conventional ADCs work by delivering a highly toxic payload to their target cells. DACs, in contrast, deliver degraders to their targets. Unlike toxins, degraders themselves have high tissue and target specificity offering an additional layer of safety that has the potential to provide DACs with a superior safety profile compared to ADCs, potentially expanding their utility beyond cancer.
Potent and Catalytic
Because degraders act catalytically to degrade multiple copies of the target protein, they possess the potency necessary for antibody delivery.
Enhanced Precision
The antibody component enables targeted delivery of degraders to specific tissues and cells of interest, and the degrader payload provides additional tissue and target selectivity.
Potential for any protein targeted, any disease treated
While we are currently focused on evaluating degrader-based medicines in cancer and autoimmune diseases, we recognize that this is just the beginning of a much larger story. Our technology is inherently disease-agnostic, holding promise for a wide range of medical conditions, and we believe that we possess the expertise and dedication to fully realize this technology’s potential.
- Cancer
- Autoimmune disease
- Metabolic disease
- Nervous system disorders