DEL-AI PLATFORM
Our innovation engine
At the heart of our innovation is our DEL-AI drug discovery engine, one of the largest and most effective targeted protein degradation platforms in the industry. This powerful engine leverages our in-house DNA Encoded Libraries (DEL), expansive collection of ligase binders, automated chemistry and direct to biology capabilities, and new insights from the application of machine learning across the platform. DEL-AI is the cornerstone of our ability to develop truly novel drugs in parallel against a broad set of targets.
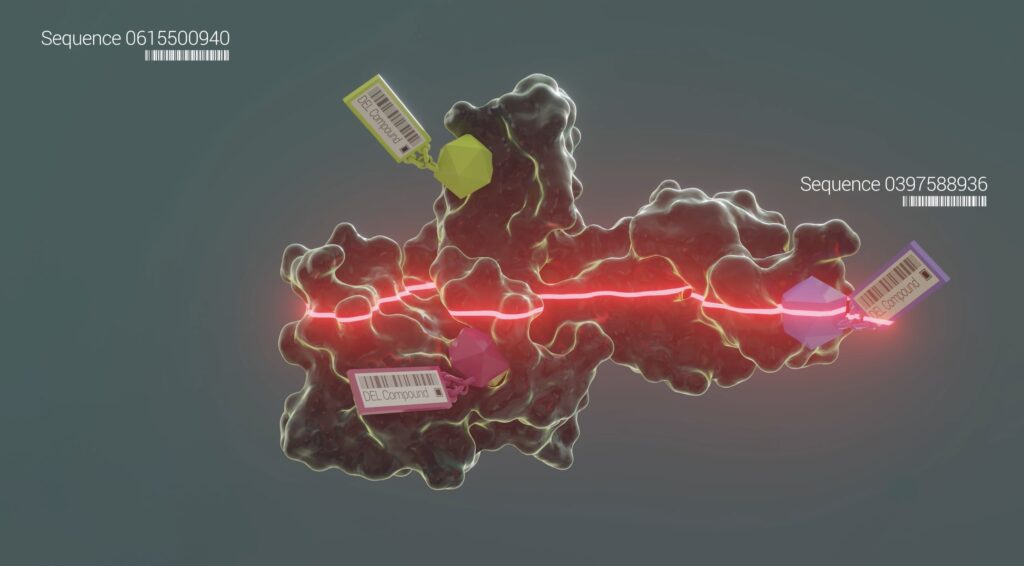
TARGETED PROTEIN DEGRADATION
Degraders and degrader antibody conjugates

We believe that targeted protein degradation (TPD) is the future of medicine. TPD is a breakthrough treatment strategy that utilizes the cell’s natural protein disposal system to eliminate disease-causing proteins. Leveraging this approach, we are advancing two innovative modalities – degraders and degrader antibody conjugates – that have the potential to overcome even the most historically intractable diseases.
RESOURCE LIBRARY
Our scientific advancements
Our collection of scientific publications, presentations, and educational materials showcases our prolific research in protein degradation. We aim to advance the entire field and deliver on the promise of degrader-based medicines to patients worldwide.
